Physicochemical properties and biocompatibility of polypyrrole-coated polycaprolactone nanofibers for guided tissue regeneration
Abstract
Polycaprolactone (PCL) nanofibers are widely used in the field of tissue regeneration as a biodegradable material. However, there is a limitation in that low hydrophilicity and tissue cannot be directly regenerated. Recent studies have shown that polypyrrole (PPy) has potential in the field of tissue engineering due to its electrical conductivity and biocompatibility. Meanwhile, the electrospinning has the advantage of using most polymers and of facilitating a porous structure suitable for tissue regeneration, so the nanofibers were fabricated through electrospinning. The purpose of this study is to evaluate the physicochemical properties and biocompatibility of PCL nanofibers coated with PPy for guided tissue regeneration. To this end, PCL nanofibers coated with four types of concentration groups were prepared. The group was named according to the concentration ratio of PPy, and the control pure PCL containing no PPy and 20PPy, 30PPy, and 40PPy containing 20 wt%, 30 wt%, and 40 wt%, respectively, consisted of the experimental group. The mixed solution of PCL and pyrrole monomer was electrospun. Then precipitate in an iron (III) chloride (FeCl3) solution as an oxidizing agent which contains pyrrole monomer and polymerized. A tensile test was performed to confirm mechanical properties, and surface hydrophilicity was confirmed through measurement of contact angle. Electrical conductivity was also confirmed through measurement of resistance values. Lastly, cytotoxicity evaluation was performed using fibroblast (L929) and preosteoblast (MC3T3-E1) cell lines to confirm biocompatibility. The results were evaluated as one-way ANOVA (p-value = 0.05), and post-analysis was performed using Tukey’s post-hoc test. PPycoated PCL nanofibers showed no statistically significant decrease in mechanical strength compared to PPy-uncoated PCL nanofibers, while electrical conductivity increased significantly at all concentrations. When 30wt% or more of PPy was coated, hydrophilicity was significantly increased compared to the PPy-uncoated PCL nanofibers. Regardless of the concentration of PPy, cytotoxicity was not shown in all groups. Accordingly, it is expected that the PPy-coated PCL fibers may be applied as a material for guided tissue regeneration. This is because of improved hydrophilicity and electrical conductivity without deteriorated physical properties and cytotoxicity.
초록
폴리카프로락톤(polycaprolactone) 나노 섬유는 생분해성 재료로 조직 재생 분야에 널리 사용되고 있다. 하지만 낮은 친수성과 조직을 직접 재생시키지 못하는 한계가 있다. 최근 연구에 따르면 폴리피롤(polypyrrole)은 전기 전도성과 생체 적합성으로 조직 공학 분야에 잠재력이 있는 것으로 나타났다. 한편 전기방사 방법은 대부분의 고분자를 이용할 수 있고, 조직 재생에 적합한 다공성 구조를 용이하게 제조할 수 있는 장점이 있어 전기방사를 통해 나노 섬유체를 제작하였다. 본 연구의 목적은 조직유도재생술을 위한 폴리피롤이 코팅된 폴리카프로락톤 나노 섬유의 물리화학적 성질과 생체적합성을 평가하는 것이다. 이를 위해 네 가지 종류 농도군의 폴리피롤이 코팅된 폴리카프로락톤 나노 섬유체를 준비하였다. 농도군은 폴리피롤의 질량비에 따라 명명되었으며, 폴리피롤이 함유되지 않은 대조군인 Pure PCL과 각각 20 wt%, 30 wt%, 40 wt%의 폴리피롤이 함유된 20PPy, 30PPy, 40PPy를 실험군으로 구성하였다. 폴리카프로락톤과 피롤 단량체 혼합 용액을 전기방사하고, 이후 피롤 단량체가 포함된 산화제로서의 염화철(III) (FeCl3) 용액에 침전하여 중합하였다. 기계적 특성을 확인하기 위하여 인장시험을 진행하였고, 접촉각 측정을 통해 표면 친수성을 확인하였다. 저항값 측정을 통하여 전기 전도성도 확인하였으며, 생체적합성을 확인하기 위해 섬유아 세포(L929)와 전조골 세포(MC3T3-E1)를 이용하여 세포독성평가를 진행하였다. 결과값은 one-way ANOVA (p-value = 0.05 기준)로 평가하였고, 사후 분석은 Tukey’s post-hoc 시험법을 이용하였다. 폴리피롤이 코팅된 폴리카프로락톤 나노 섬유체는 코팅되지 않은 폴리카프로락톤 나노 섬유체에 대비하여 기계적 강도의 통계적으로 유의미한 감소는 없었던 반면 모든 농도에서 전기 전도성이 유의미하게 증가했다. 30 wt% 이상의 폴리피롤이 코팅되었을 때, 코팅되지 않은 폴리카프로락톤 나노 섬유체 대비 친수성이 유의미하게 증가했으며, 폴리피롤의 농도와 관계없이 모든 군에서 세포독성을 보이지 않았다. 이에 폴리피롤이 코팅된 폴리카프로락톤 나노 섬유체는 물성 및 세포독성의 감소 없이 향상된 친수성과 전기 전도성을 통해, 조직유도재생술을 위한 재료로 적용할 수 있을 것으로 기대한다.
Keywords:
Dental materials, Guided tissue regeneration, Electrospinning, Polypyrrole, Conductive scaffold키워드:
치과재료, 조직유도재생술, 전기방사, 폴리피롤, 전도성 지지체Introduction
Various bacteria inhabit the human oral condition, and these bacteria lead to periodontal diseases and various systemic diseases (acute endocarditis, etc.), which deteriorate the quality of life (1). Therefore, it is essential to control bacteria in the treatment and surgery of oral diseases. Polymeric dental materials in the maxillary area are drug-based chemical approaches that have focused on symptom relief and prevention rather than the removal of fundamental causes (2). The strong conductivity of polypyrrole (PPy) has bactericidal activity. It performs the function of sterilization by directly destroying the cell wall of the bacteria or by interfering with the proton balance through interactions with the cell membrane of the bacteria (3). Therefore, we noted the clinical potential of PPy as a material for bacterial control. Therefore, this study evaluated the application of PPy, a polymer having electrical conductivity.
Meanwhile, in the field of dentistry, guided tissue regeneration (GTR) technology is widely used, which is a technique to secure temporal and physical space for hard tissue regeneration by preventing the proliferation of soft tissue. Our goal is to create improved dental materials by adding new specifications to the membrane that plays a key role in GTR technology. We tried the electrical approach from the GTR membrane by applying the electrical conductivity of PPy. There are a few Research have shown that inducing tissue regeneration through electrical stimulation promotes non-invasive differentiation of tissue cells (4, 5). This subject has been studied in various fields of nerve regeneration. Recently, the possibility of utilizing electrical stimulation in hard tissue regeneration is being explored, but the development and application of highly reliable electrically conductive polymers with biocompatibility remains a challenge (6). Based on these studies, the goal of our study is to lay the foundation from a dental material perspective for application in dental clinical practice. This is to study differentiation at the cell level in GTR through electrical energy.
The fabrication of nanofibers through electrospinning creates a layered structure. The microstructure with porosity and roughness, which is used to fabricate materials for bone regeneration by increasing the available surface area for cell attachment and growth (7). In addition, biodegradable materials, including polycaprolactone (PCL), has already been used in various fields of tissue engineering, since it has obtained Food and Drug Administration (FDA) approval as a GTR membrane due to their durable mechanical properties with biocompatibility and flexibility (8). However, the properties of hydrophobicity and slow decomposition rate are pointed out as disadvantages of PCL (8, 9). Hydrophobic properties are disadvantageous in cell attachment and penetration. To overcome the lack of hydrophilicity of PCL, attempts have been made through surface modification, mixing with hydrophilic polymers, and adding hydrophilic filters have been (10-12). As an extension of these efforts, we try to overcome the limitations of hydrophobicity of PCL through surface modification using PPy and surfactants.
Polypyrrole is a biodegradable polymer with electrical conductivity and has biocompatibility itself. Hence, it is a material that stimulates cell differentiation of nerve and muscle systems and is attracting attention in the field of tissue engineering using electrical stimulation (13, 14). The development of materials using PPy in the field of hard tissue regeneration has recently been carried out. Therefore, specific research in the oral maxillary area using PPy is especially rare. Through this study, we intend to develop a material with electrical conductivity by overcoming the hydrophobicity of PCL to form a surface favorable for cell proliferation and attachment and using PPy.
Materials and Methods
1. Materials
Biodegradable polymers PCL (Sigma Aldrich, St. Louis, MO), dichloromethane (DCM, Sigma Aldrich, Darmstadt, Germany), and N, N-dimethylformamide (DMF, Sigma Aldrich, Darmstadt, Germany) were used as base material, and pyrrole (FeCl3, Duksan Pure Chemical, Seoul, South Korea) with 2-naphthalene sulfonate (2-NS, Sigma Aldrich, Darmstadt, Germany) which was used as surfactants. Distilled water and methanol (Fisher Scientific, Hampton, NH) as solvents were used to polymerize pyrrole. 2-propanone (Samchun Pure Chemical, Pyeongtaeksi, South Korea) was used for washing the prepared nanofibers.
2. Fabrication of nanofibers by electrospinning
Polycaprolactone was dissolved in a solvent of DCM and DMF at a volume ratio of 4:6 and mixed so that the final concentration was 12 wt%. First, PCL was mixed with DCM, stirred for 1 h, and then stirred for 3 h by adding DMF to prepare a 12 wt% PCL mixed solution. Pyrrole monomers were added to the mixed solution at 20, 30, and 40 wt%, respectively, according to Table 1, and referred to as 20PPy, 30PPy, and 40PPy, respectively.
Electrospinning/Electrospray system (ESR200R2, eSrobot, NanoNC, Seoul, South Korea) was used for electrospinning. Electrospinning was performed around a rotating drum at a speed of 450 rpm for 2.5 h to fabricate nanofibers. Flow rate of the prepared solution was 1.0 mL/h with a voltage of 15.0 kV, and the distance between the 21G needle and the collector was 150 mm. After the manufacture, each specimen was prepared by cutting into 80×80 mm2.
The prepared nanofibers layer was polymerized after being immersed in a polymerization solution at 4 ℃ for 4 h. Agents and samples required for polymerization were prepared according to Table 2. After mixing 180 mL of distilled water and 60 mL of methanol, pyrrole monomer and 2-NS, a surfactant, were added and mixed at 450 rpm for 15 min in a magnetic stirrer. Then, FeCl3, which is an oxidizing agent, was dissolved in 60 mL of distilled water. The nanofibers layer was immersed in the mixture, and then the immediately dissolved FeCl3 solution was added and polymerized at 4 ℃ for 4 h. After completion of the polymerization, the nanofibers were washed five times for 1 min with acetone and overnight dried in a fume hood at room temperature.
3. Setting optimum conditions for electrospinning
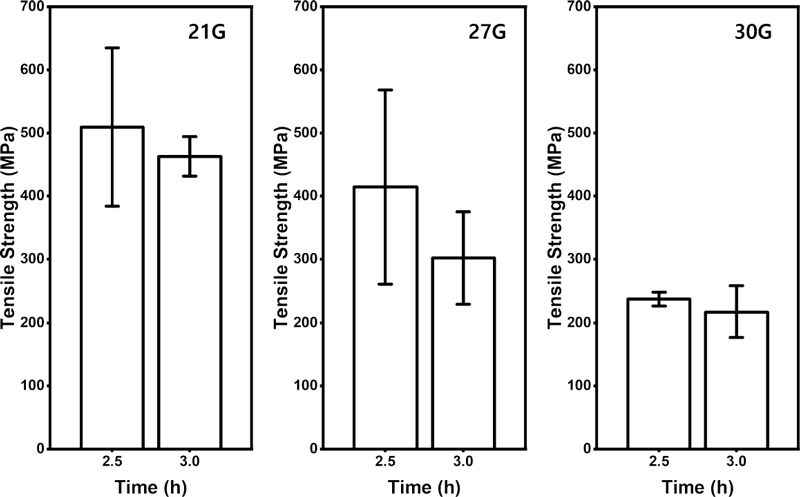
The mechanical properties and surface shape of the nanofibers produced by electrospinning are affected by applied voltage, solution viscosity and release rate, rotational speed of the collector, the distance between the collector and the syringe tip, temperature, and humidity (15, 16). Other than these known variables, it was found that the mechanical properties and surface shape of the nanofibers experimentally affected by other factors. Such variables, size and spinning duration of the needle gauge produce different nanofibers structure in this study. Accordingly, optimal conditions of the electrospinning were found by setting the necessary gauge and the radiation time as variables. Figure 1 shows the average fibers diameter and standard deviation of fibers produced by the necessity gauge and the radiation time. The surface of the fibers was measured through field emission-scanning electron microscope (FE-SEM, JSM-IT700HR, Jeol, Tokyo, Japan) and the fibers diameter was measured through ImageJ software (National Institute of Health, Bethesda, MD). Twenty-five individual fibers were selected to measure the diameter in the image. After 2.5 to 3 h of the spinning time, the standard deviation was relatively low and there was little difference. The low standard deviation can be interpreted as uniform fibers diameter. Accordingly, the tensile strength of 2.5 h and 3 h for each gauge was measured with four samples respectively, and it was expressed as Figure 2. There was no significant difference in the tensile strength over spinning time in each gauge, but all gauges showed that the intensity increased with shorter spinning time. When the needle gauge was 21G and the spinning time was 2.5 h, the highest tensile strength value was shown, which was 509.3 MPa. Therefore, electrospinning was carried out in consideration of this as an optimal condition for electrospinning.
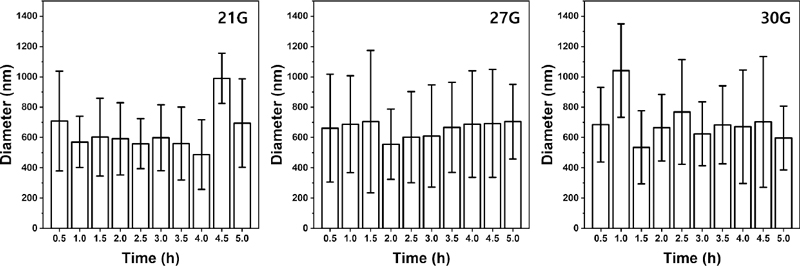
Mean diameter and standard deviation of electrospun 12 wt% PCL nanofibers by needle gauge (21G, 27G, and 30G) and spinning time (0.5–5.0 h)
4. Measurement of surface and chemical properties
The surface structure and shape of the nanofibers produced by electrospinning were observed with a FE-SEM. In addition, fourier transform infrared (FTIR, Vertex 70, Bruker, Billerica, MA) spectroscopy analysis was performed to confirm the presence of PPy in PPycoated PCL nanofibers. The contact angle was measured using (room temperature, 45% humidity) saline solution was used, and three specimens (20×20 mm2) for each concentration were prepared and measured.
5. Measurement of physical properties
Tensile strength was measured using a universal testing machine (UTM, Instron 3367, Norwood, MA), and six specimens [20 (w)×60 (l) mm2] for each concentration were prepared. The zig of UTM was located at 20×20 mm2 at both ends of the specimen. Electrical conductivity was analyzed through resistance value measurement. A digital multimeter (600V CAT III Digital multimeter with temperature and frequency, Fluke, Everett, WA) was used, and the distance between the two electrodes was set to 10 mm. Each specimen was measured six times.
6. In vitro tests
MC3T3-E1 and L929 were cultured in α-MEM (Welgene, Gyeongsan-si, South Korea) for MC3T3-E1 and RPMI 1640 (Welgene, Gyeongsan-si, South Korea) for L929 supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY) and 100 μg/mL antibiotic/antimycotic mix at 37 ℃ in an atmosphere with 95% humidity and 5% CO2.
To confirm the biocompatibility of the specimen, the specimen eluate test method was performed with reference to ISO 10993-5 (17). As positive and negative controls (PC and NC), natural latex rubber and polyethylene film were used respectively. Cell proliferation was quantitatively analyzed using the WST assay (EZ-Cytox, DoGenBio, Seoul, South Korea) in accordance with the method described in a manual. The optical density was measured at 450 nm using a microplate spectrophotometer (Epoch, Bio-Tek, Seoul, South Korea).
The cell viability of the nanofibers was analyzed using the LIVE/DEAD viability/cytotoxicity kit (Invitrogen, Carlsbad, CA). In brief, MC3E3-E1 cells were incubated in eluate of the nanofibers for three days and washed with PBS. The cells on the plate were then stained in LIVE/DEAD reagents for 30 min. The stained cells were then observed by confocal laser scanning microscopy (LSM 700, Zeiss, Oberkochen, Germany) to assess the cell viability.
7. Statistical analysis
Experimental results were statistically analyzed by one-way ANOVA (SPSS 23, Chicago, IL). Post test was performed by the Tukey’s post-hoc test, and the significance level was set to 0.05.
Results
1. Surface structures
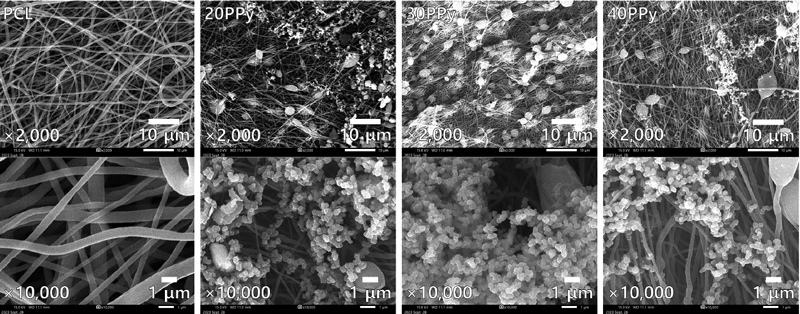
Figure 3 shows the representative SEM images of the nanofibers, which are observed at 2,000 and 10,000 magnifications. After the platinum coating on the electrospun nanofibers, the surface shape could be secured because of measuring at 10 kV of an acceleration voltage using FE-SEM (JSM-IT700HR, Jeol, Tokyo, Japan). Through the SEM images, randomly aligned PCL nanofibers and PPy coatings were detected, and the electrospun PCL nanofibers diameter was 709 ± 329 nm.
2. Chemical properties
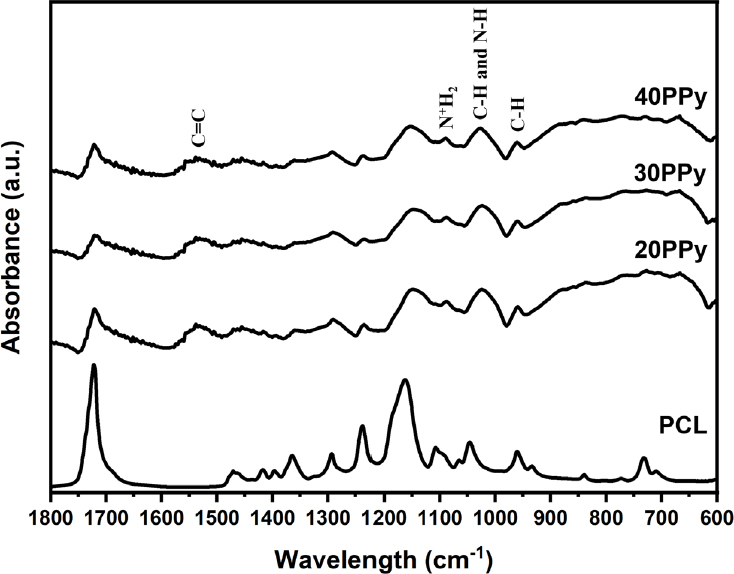
To confirm the presence of PPy in the PCL nanofibers, the surfaces of six random samples nanofibers for each group were analyzed using FTIR. Figure 4 shows the FTIR spectrum of pure PCL nanofibers and PPy-coated nanofibers. The surface of the nanofibers (20PPy, 30PPy, and 40PPy) to which PPy was added showed a peak distinguished from PCL, which is 1558 to 1541 cm-1 corresponding to C=C stretching vibration of the pyrrole ring, and 1090 cm-1 corresponding to N+H2 vibration of the PPy ring, 1035 cm-1 of the corresponding C-H and N-H vibration of the pyrrole ring, and 960 cm-1, which means C-H vibration, were observed.
3. Mechanical properties
Yield strength, ultimate strength, and modulus of elasticity for each group are presented in Figure 5. Using UTM, the stress-strain curves were calculated with six random samples for each group and mechanical properties were calculated. With the increase of PPy, the yield strength and the ultimate strength were decreased in general, and the modulus of elasticity was increased in general. The yield strength of PCL, 20PPy, 30PPy, and 40PPy were 48.03 ± 5.40, 44.43 ± 7.48, 28.16 ± 8.44, and 32.86 ± 21.65 MPa, respectively. The ultimate strength of PCL, 20PPy, 30PPy, and 40PPy groups were 176.66 ± 20.53, 146.83 ± 38.65, 52.05 ± 22.45, and 105.84 ± 77.93 MPa, respectively. In the same order, the data of the modulus of elasticity were 706.37 ± 87.16, 966.92 ± 168.55, 753.24 ± 240.14, and 1429.17 ± 812.53 MPa, respectively.
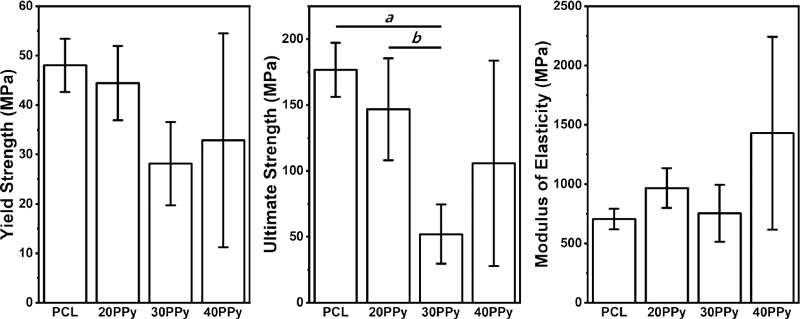
Mechanical properties (yield strength, ultimate strength, and modulus of elasticity) of pure PCL nanofiber and PPy-coated PCL nanofibers. ‘a’ means that there is a statistically significant difference between PCL and 30PPy, and ‘b’ means that there is a statistically significant difference between 20PPy and 30PPy.
At 30PPy, a significant decrease in ultimate strength was confirmed compared to pure PCL (p = 0.002) and 20PPy (p = 0.019), but within the yield strength and the modulus of elasticity, there were no significant difference between all groups. Through this, it is considered to have sufficient mechanical properties to function as a shielding film for GTR that the material of this experiment has in mind.
4. Contact angle
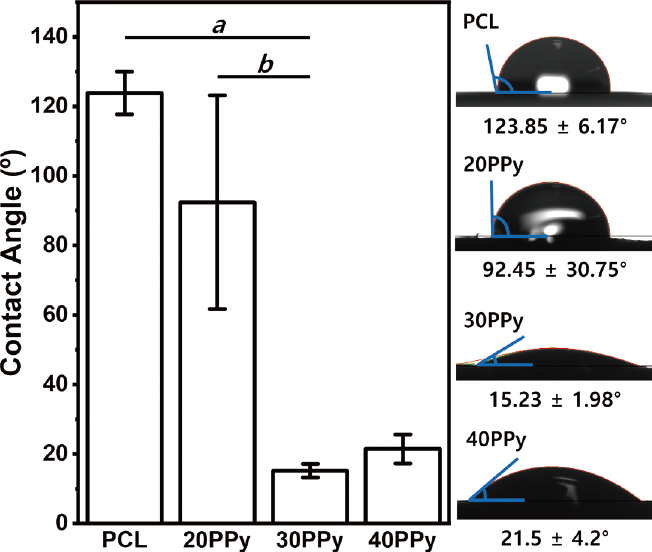
The contact angle was measured for five random samples for each group using saline solution. For the sample showing a gradually absorbed pattern, it was confirmed based on the contact angle after two second after surface contact. The contact angles are generally increased with concentration. For pure PCL, 20PPy, 30PPy, and 40PPy, contact angles were 123.85 ± 6.17, 92.45 ± 30.75, 15.23 ± 1.98, and 21.5 ± 4.2°, respectively, as shown in Figure 6. Whereas 30PPy and 40PPy showed hydrophilic performance (θ < 90°), pure PCL and 20PPy showed hydrophobic performance (θ > 90°). The contact angle of nanofibers showed a significant increase at 30PPy compared to pure PCL and 20PPy (p < 0.001). There was no significant difference in contact angle between pure PCL and 20PPy (p = 0.072). Likewise, there was no significant difference in contact angle between 30PPy and 40PPy (p = 0.209).
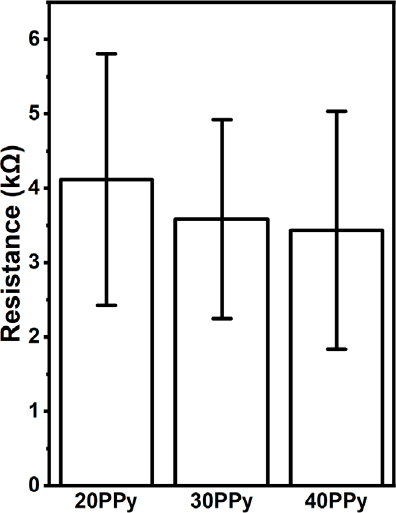
5. Electrical conductivity
The electrical conductivity measurement was performed through the electrical resistance value measurement. The electrical resistance values were measured by measuring five random samples in each group except pure PCL and shown in Figure 7. The pure PCL nanofibers perform high electrical resistance, and the value was so high that it could not be measured with our measuring equipment. For 20PPy, 30PPy, and 40PPy, the electrical resistances were 4.1 ± 1.7, 3.6 ± 1.31, and 3.4 ± 1.6 kΩ, respectively. Using the correlation between the surface resistance and electrical conductivity, the electrical conductivity of the nanofiber was indirectly confirmed (18, 19). As a result, it was confirmed that the electrical resistance decreased proportionally as the PPy concentration increased, suggesting that the presence of PPy on the nanofibers enhances the electrical conductivity. Through this, the material produced through the PPy coating process has electrical conductivity. This is thought to have meaning in conducting cell-level research through electrical stimulation.
6. Biocompatibility
To evaluate the impact of PPy coating with PCL nanofibers on biocompatibility, WST assay and LIVE/ DEAD assays were performed to assess cell proliferation and viability.
WST assays were conducted at mouse-derived fibroblasts (L929) and preosteoblasts (MC3T3-E1), which are demonstrated as Figure 8(A). As a result, the cell viability of the experimental groups showed no significant difference to that of the negative control group. This could be confirmed in all groups, which contrasts clearly with the positive control group. Through this, it may be seen that the experimental groups have biocompatibility that may be potentially applied in the field of dental materials and may be used in bone and epithelial tissues.
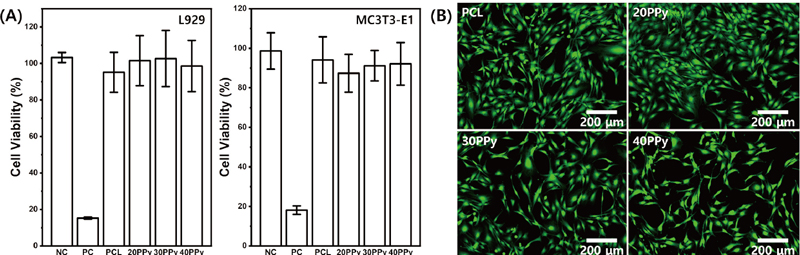
(A) Cytotoxicity of pure PCL nanofibers and PCL nanofibers coated with PPy in L929 and MC3T3-E1 cells by WST assay. (B) Fluorescence microscopy images of pure PCL nanofibers and PPy-coated PCL nanofibers in MC3T3-E1 by LIVE/DEAD assay. The live and dead cells exhibited green and red fluorescence, respectively.
LIVE/DEAD Assay using MC3T3-E1 was conducted to verify the biocompatibility. As shown in Figure 8(B), no change in cell morphology was observed in all groups, and live cells were prominent, confirming the biocompatibility of PCL nanofibers and PPy-coated PCL nanofibers.
Discussion
The topography of the PCL nanofibers was characterized using SEM. The electrospun nanofibers were observed as a chain-like shape, and nanoparticles were randomly distributed around the nanofibers. As the concentration of PPy increased, a significant change in surface topology was not observed. The PPy observed on the SEM showed one pattern of pores, grooves, and jagged, and it has been reported that this structure is advantageous for cell proliferation compared to a smooth surface. The characteristics of the surface depend on the ligand molecule of the cell present on the surface, the secretion of protein, and polarity, which is deeply related to the increase in contact area as the roughness increases (20). The surface energy of the polymer produced by electrospinning is affected by the diameter and density of the fibers serving as a support for the formed fibers (15). This is affected by the needle gauge and time, as can be seen in Figure 1. Therefore, it is expected that the contact area will increase by the PCL nanofibers themselves rather than the presence and concentration increase of PPy itself. The fact that a significant increase in surface porosity was not observed with an increase in PPy in the FE-SEM shape histogram analysis results using ImageJ also supports this inference.
In the FTIR performed to confirm the vesicular particles observed on the surface, the presence of PPy on the surface of the nanofibers (20PPy, 30PPy, and 40PPy) to which PPy was added was confirmed by the FTIR. The presence of PPy on the surfaces of all experimental groups was confirmed by the unique values of PPy-added nanofibers (20PPy and 30PPy) which is 1558 to 1541 cm-1 corresponding to C=C stretching vibration of the pyrrole ring, 1090 cm-1 corresponding to the N+H2 vibration of the PPy chain, and 1035 cm-1 corresponding to the C-H vibration of the pyrrole ring. This peak is a unique value of PPy, which is distinguished from pure PCL, and the presence of PPy on the surfaces of all experimental groups (21).
At 30PPy, a statistically significant decrease in tensile strength was confirmed in terms of maximum strength, but it was confirmed that it had a mechanical strength like that of the control group overall. Nevertheless, the tensile strength showing 30PPy is not significantly inferior when compared with the tensile strength of most GTR membrane currently available on the market. According to previous studies, the ultimate strength of the collagen membrane of three commercially available companies (Bio-Gide, Collprotect, and Jason) did not exceed 25 MPa (22). In this respect, the value of 50 MPa shown by 30PPy still means that it has sufficient mechanical properties that can be used without difficulty in clinical practice. In addition, it has been reported that the ultimate strength of a GTR membrane with enhanced mechanical properties such as tensile strength (e.g., a polymer loaded with beta-tricalcium phosphate) is 200 MPa (23). The ultimate strength of the 20PPy, that was identified in this experiment, is an excellent level of mechanical strength considering the results of this study. In this respect, it is considered that the physical properties shown by the experimental group are good.
As shown in Figure 5, the overall tensile strengthrelated index showed some decrease as the concentration of PPy increased. According to previous studies, as the concentration of pyrrol monomers in the PCL/PPy complex increased, fibers with a large diameter (400–500 nm) were drastically decreased and fibers with a small diameter (400 nm or less) were decreased (24). This study did not mention the effect of this overall change in fibers diameter on tensile strength. In this study, it is presumed that the decrease in field strength and ultimate strength is a result of the decrease in fiber diameter.
The surface hydrophilicity of the produced nanofibers in terms of hydrophilicity showed a statistically significant increase in hydrophilicity at 30PPy and 40PPy compared to the control group, and there was no significant difference between 30PPy and 40PPy. The cause of the increase in hydrophilicity is thought to be the effect of the action of 2-NS added as a surfactant in the polymerization process. Liu Y et al. reported that the mixing of 2-NS and the oxidizing agent FeCl3 is made into a hydrophilic phase, and pyrrole is mixed with methanol to form an oil phase to change the hydrophilic-lipophilic balance (HLB) of the PCL and PPy mixture (25). We suppose the reason that the surface hydrophilicity, which did not increase significantly at 20PPy, reached 30PPy is that a threshold for uniformly affecting the entire nanofibers exist. Further study is needed to verify the influence of surfactants in the coating and polymerization process of PPy. The increase in electrical conductivity, which can be seen as a significant increase in the surface resistance value, shows the possibility of clinical application of electrical energy in the field of regeneration revealed through several previous studies (21, 26). Tissue regeneration at the celllevel was not confirmed in our study. This is expected to be confirmed through future experiments.
Conclusions
The purpose of this study is to produce a GTR membrane with electrical conductivity through PPy. It also aims to improve the hydrophobicity, the limit of PCL, through surfactants added during the manufacturing process. Our experimental results are summarized as follows by substituting the experimental group thus produced for each of the ideal GTR membrane conditions and comparing them.
Biocompatibility, biodegradability, good mechanical properties, and surface characteristic/morphology are suggested as ideal conditions for the GTR shielding membrane generally used in tissue engineering (27-29). Based on these criteria, it meets the first criteria, biocompatibility. the materials tested in this study confirmed the absence of toxicity according to ISO 10993-5 and the level of biocompatibility applied to bone/epithelial tissue, as revealed by the cytotoxicity test (WST assay and LIVE/DEAD assay). The second criteria, biodegradability of PCL and PPy alone, has been verified through several studies. The third criteria, Adequate mechanical properties, was found to have good mechanical properties in that there was no significant difference between the currently commercially available pure PCL in terms of tensile strength measurements and elastic modulus calculated based on stress/strain curves (30). As observed by FE-SEM, the surface shape that is easy for cell migration and attachment. The uniform porous diameter structure of nanofibers, which is made by electrospinning, can serve as a substrate for cell migration and attachment. Furthermore, as can be seen from the measurement of contact angle, the biomaterial produced through a polymerization process above a certain level has higher surface hydrophilicity. Compared to the control group, this shows an environmental advantage in cell growth. Therefore, it also meets the fourth criterion, surface characteristic/morphology.
The clinical significance of this study is that it studied the material properties that can utilize electrical energy to regenerate the tissue defect. A typical example is the electrical conductivity of the PPy-coated PCL nanofiber that we confirmed through experiments.
Acknowledgments
This study was supported by 2022 Academic Research Support Program in Gangneung-Wonju National University, and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by Korea Government (MSIT) (No. RS-2023-00210838).
References
-
Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117(24):3118-25.
[https://doi.org/10.1161/CIRCULATIONAHA.107.758524]

-
Tahmasebi E, Keshvad A, Alam M, Abbasi K, Rahimi S, Nouri F, et al. Current Infections of the Orofacial Region: Treatment, Diagnosis, and Epidemiology. Life (Basel). 2023;13(2).
[https://doi.org/10.3390/life13020269]

-
da Silva FA, Jr., Queiroz JC, Macedo ER, Fernandes AW, Freire NB, da Costa MM, et al. Antibacterial behavior of polypyrrole: The influence of morphology and additives incorporation. Mater Sci Eng C Mater Biol Appl. 2016;62:317-22.
[https://doi.org/10.1016/j.msec.2016.01.067]

-
Gordon T, Chan KM, Sulaiman OA, Udina E, Amirjani N, Brushart TM. Accelerating axon growth to overcome limitations in functional recovery after peripheral nerve injury. Neurosurgery. 2009;65(4 Suppl):A132-44.
[https://doi.org/10.1227/01.NEU.0000335650.09473.D3]

-
Barber B, Seikaly H, Ming Chan K, Beaudry R, Rychlik S, Olson J, et al. Intraoperative Brief Electrical Stimulation of the Spinal Accessory Nerve (BEST SPIN) for prevention of shoulder dysfunction after oncologic neck dissection: a double-blinded, randomi zed controlled trial. J Otolaryngol Head Neck Surg. 2018;47(1):7.
[https://doi.org/10.1186/s40463-017-0244-9]

- Khare D, Basu B, Dubey AK. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials. 2020;258:120280.
-
Xue J, Wu T, Dai Y, Xia Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem Rev. 2019;119(8):5298-415.
[https://doi.org/10.1021/acs.chemrev.8b00593]

-
Bharadwaz A, Jayasuriya AC. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110:110698.
[https://doi.org/10.1016/j.msec.2020.110698]

-
Fabbri P, Bondioli F, Messori M, Bartoli C, Dinucci D, Chiellini F. Porous scaffolds of polycaprolactone reinforced with in situ generated hydroxyapatite for bone tissue engineering. J Mater Sci Mater Med. 2010;21(1):343-51.
[https://doi.org/10.1007/s10856-009-3839-5]

-
Gorna K, Gogolewski S. The effect of gamma radiation on molecular stability and mechanical properties of biodegradable polyurethanes for medical applications. Polymer Degradation and Stability. 2003;79(3):465-74.
[https://doi.org/10.1016/S0141-3910(02)00362-2]

-
Ehtesabi H, Massah F. Improvement of hydrophilicity and cell attachment of polycaprolactone scaffolds using green synthesized carbon dots. Materials Today Sustainability. 2021;13:100075.
[https://doi.org/10.1016/j.mtsust.2021.100075]

-
Khoee S, Kardani M. Preparation of PCL/PEG superporous hydrogel containing drug-loaded nanoparticles: the effect of hydrophobic–hydrophilic interface on the physical properties. European polymer journal. 2014;58:180-90.
[https://doi.org/10.1016/j.eurpolymj.2014.06.024]

-
Uzieliene I, Popov A, Vaiciuleviciute R, Kirdaite G, Bernotiene E, Ramanaviciene A. Polypyrrole-based structures for activation of cellular functions under electrical stimulation. Bioelectrochemistry. 2024;155: 108585.
[https://doi.org/10.1016/j.bioelechem.2023.108585]

-
Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Baharvand H, Kiani S, et al. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J Tissue Eng Regen Med. 2011;5(4):e17-35.
[https://doi.org/10.1002/term.383]

-
Chinnappan BA, Krishnaswamy M, Xu H, Hoque ME. Electrospinning of Biomedical Nanofibers/ Nanomembranes: Effects of Process Parameters. Polymers (Basel). 2022;14(18).
[https://doi.org/10.3390/polym14183719]

-
Lian H, Meng Z. Melt electrospinning vs. solution electrospinning: A comparative study of drug-loaded poly (epsilon-caprolactone) fibres. Mater Sci Eng C Mater Biol Appl. 2017;74:117-23.
[https://doi.org/10.1016/j.msec.2017.02.024]

- International Organization for Standardization. ISO 10993-5:2009. Biological evaluation of medical devices, Part 5: Tests for in vitro cytotoxicity. Geneva: ISO; 2009.
-
Folorunso O, Hamam Y, Sadiku R, Ray SS, Joseph AG. Parametric Analysis of Electrical Conductivity of Polymer-Composites. Polymers (Basel). 2019;11(8).
[https://doi.org/10.3390/polym11081250]

-
Mohammadpour-Haratbar A, Zare Y, Rhee KY. Simulation of electrical conductivity for polymer silver nanowires systems. Sci Rep. 2023;13(1):5.
[https://doi.org/10.1038/s41598-022-25548-w]

-
Majhy B, Priyadarshini P, Sen AK. Effect of surface energy and roughness on cell adhesion and growth - facile surface modification for enhanced cell culture. RSC Adv. 2021;11(25):15467-76.
[https://doi.org/10.1039/D1RA02402G]

-
Runge MB, Dadsetan M, Baltrusaitis J, Knight AM, Ruesink T, Lazcano EA, et al. The development of electrically conductive polycaprolactone fumaratepolypyrrole composite materials for nerve regeneration. Biomaterials. 2010;31(23):5916-26.
[https://doi.org/10.1016/j.biomaterials.2010.04.012]

- Ortolani E, Quadrini F, Bellisario D, Santo L, Polimeni A, Santarsiero A. Mechanical qualification of collagen membranes used in dentistry. Ann Ist Super Sanita. 2015;51(3):229-35.
-
Abtahi S, Chen X, Shahabi S, Nasiri N. Resorbable Membranes for Guided Bone Regeneration: Critical Features, Potentials, and Limitations. ACS Mater Au. 2023;3(5):394-417.
[https://doi.org/10.1021/acsmaterialsau.3c00013]

-
Maharjan B, Kaliannagounder VK, Jang SR, Awasthi GP, Bhattarai DP, Choukrani G, et al. In-situ polymerized polypyrrole nanoparticles immobilized poly(epsilon-caprolactone) electrospun conductive scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2020;114:111056.
[https://doi.org/10.1016/j.msec.2020.111056]

-
Liu Y, Wu F. Synthesis and application of polypyrrole nanofibers: a review. Nanoscale Adv. 2023;5(14):3606- 18.
[https://doi.org/10.1039/D3NA00138E]

-
Hosoyama K, Ahumada M, Goel K, Ruel M, Suuronen EJ, Alarcon EI. Electroconductive materials as biomimetic platforms for tissue regeneration. Biotechnol Adv. 2019;37(3):444-58.
[https://doi.org/10.1016/j.biotechadv.2019.02.011]

-
Yang Z, Wu C, Shi H, Luo X, Sun H, Wang Q, et al. Advances in Barrier Membranes for Guided Bone Regeneration Techniques. Front Bioeng Biotechnol. 2022;10:921576.
[https://doi.org/10.3389/fbioe.2022.921576]

-
Toledano-Osorio M, Manzano-Moreno FJ, Ruiz C, Toledano M, Osorio R. Testing active membranes for bone regeneration: A review. J Dent. 2021;105:103580.
[https://doi.org/10.1016/j.jdent.2021.103580]

-
Feng P, He R, Gu Y, Yang F, Pan H, Shuai C. Construction of antibacterial bone implants and their application in bone regeneration. Mater Horiz. 2023.
[https://doi.org/10.1039/D3MH01298K]

-
Wang J, Wang L, Zhou Z, Lai H, Xu P, Liao L, et al. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers (Basel). 2016;8(4).
[https://doi.org/10.3390/polym8040115]